What are inorganic Ions?
Nitrate
Phosphate
Introduction
Inorganic ions like nitrate (NO3–), calcium (Ca2+), hydrogen carbonate (HCO–3), potassium (K+), iron (Fe), magnesium (Mg2+) and phosphate (PO43-) are key components of molecules in living things. Here are a few examples of where they can be found and what their role is within plants.
Nitrate
Nitrate ions are extracted by plants from the soil, and their nitrogen atoms used for other things. There are of course the nitrogenous bases in DNA (adenine, guanine, cytosine and thymine) as well as amino acids – hence amino acids. Get it get it.
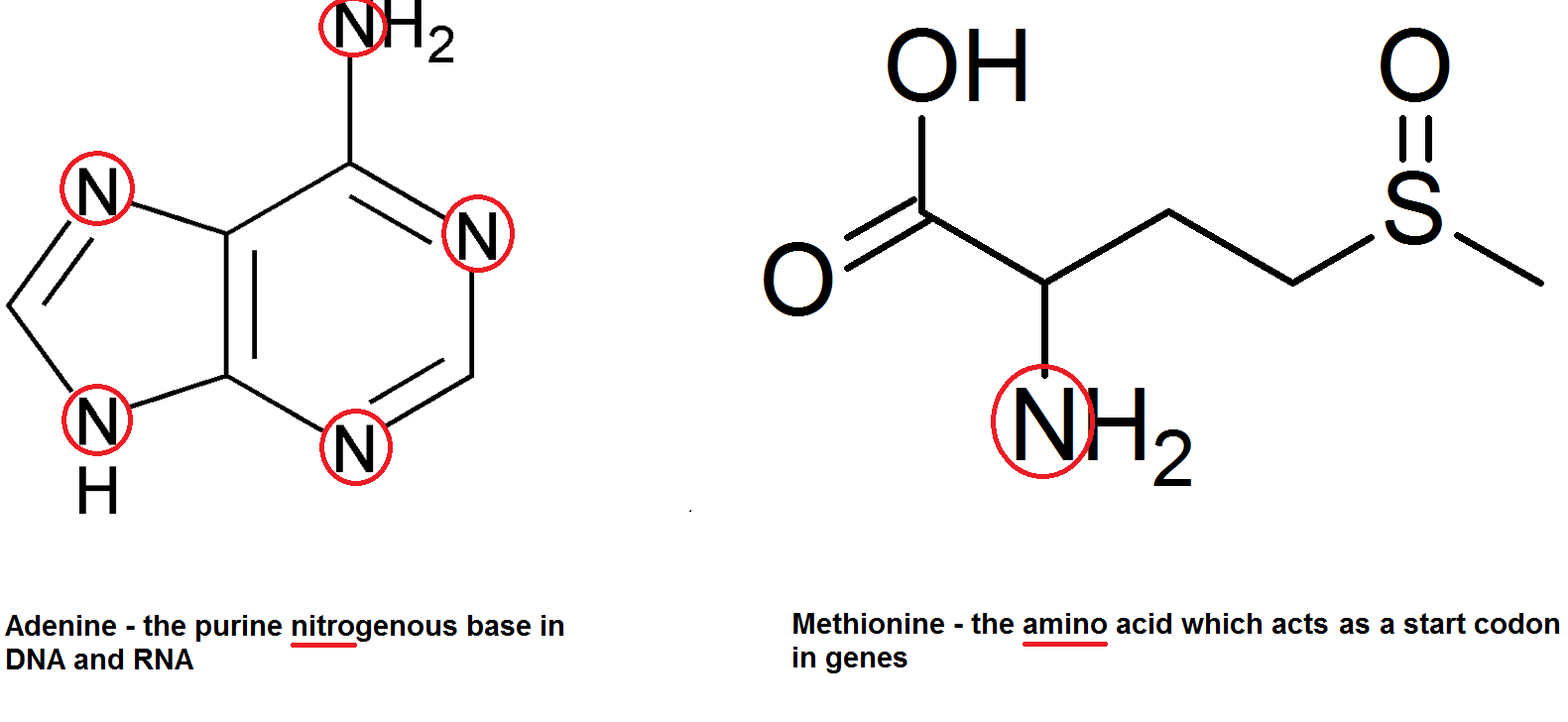
This of course applies to all life since all life does have DNA (or RNA) and amino acids.
Calcium ions have a key role in calcium pectate which I have just googled. I’m afraid what I found is far too hilarious to ignore:
“Calcium pectate, a pectin fiber that adds crispness to fruits and vegetables, also has potent cholesterol-lowering properties.”
