What are carbohydrates made of?
What is the structure of carbohydrates?
Carbohydrates (including sugars) are made of carbon, hydrogen and oxygen. They come in various sizes and chemical arrangements and hence serve multiple functions in biology, including energy storage and structural support.
The smallest units (monomers) of carbohydrates are simple sugars and include trioses, pentoses and hexoses, so named due to the number of carbon atoms present (3, 5 and 6 respectively). They are monosaccharides.
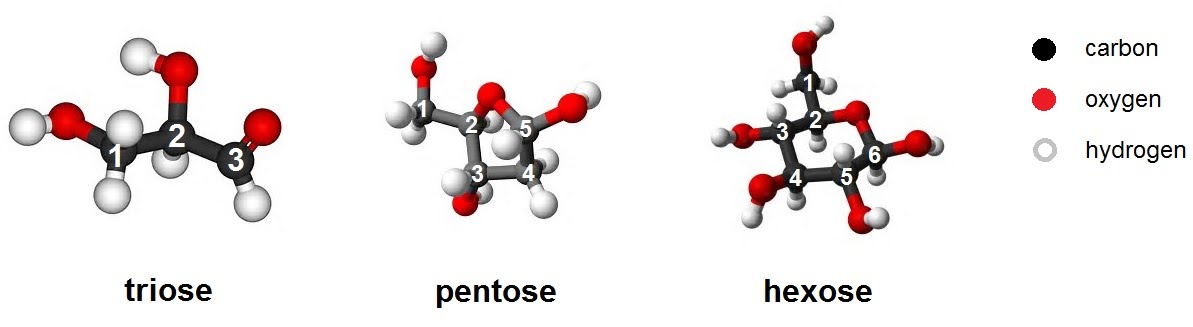
The triose glyceraldehyde for example, is an intermediate in the metabolism of carbohydrates to produce energy during cellular respiration. The pentose deoxyribose is a constituent of no other than DNA itself, while the hexose glucose needs no introduction as the central energy processing molecule created in photosynthesis and expended in respiration.
Glucose is a key carbohydrate in biology because it is the preferred fuel that provides energy during cellular respiration as well as the building block for complex polysaccharides and other compounds that use it such as glycoproteins. As a monomer, it is small and water soluble and thus accessible to cells for respiration. Plants and other organisms can create it through photosynthesis. It can be built into starch or glycogen and stored away for later use. When required, it can be obtained by breaking starch or glycogen down again. This is what maintains…
