Since enzymes are so specific, powerful – many thousands of times faster than some chemical catalysts – and operate at much lower temperatures, too, they are employed in many industries including food and cleaning.
These industries work with tremendous amounts of product, so the amount of enzyme used for each reaction would be equally huge and very uneconomical, as enzymes can be expensive to make. Imagine adding a lot of precious enzymes to a giant processor of, say, baby food or detergent. You’d like to reuse the expensive enzymes, but you can’t extract the enzyme back from the giant processor full of stuff.
This is why enzymes are immobilised onto a surface or tool which is used for processing the product instead. This makes it easy to reuse the enzymes and improves the stability of their reactions. How may the enzymes be immobilised?
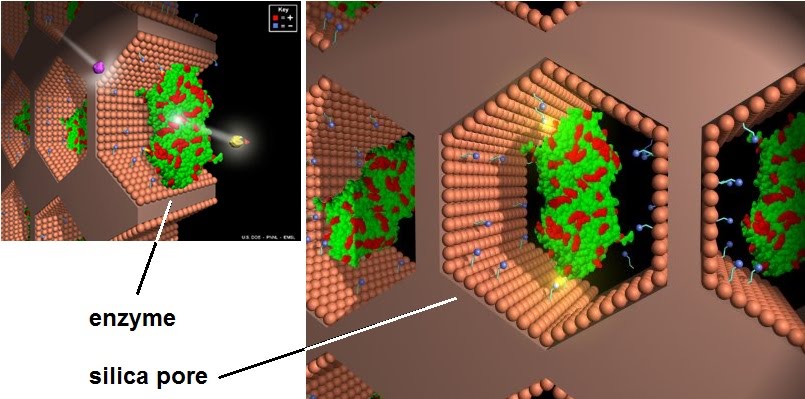
They can be fancily bound chemically to smaller attachment chemicals (small blue spheres) inside nano-sized silica pores, or more conventionally stuck inside semi-permeable jelly balls of…
