What is Osmosis?
When does Osmosis occur?
Isotonic
What is Osmosis?
Osmosis is the diffusion of water across a semi-permeable membrane. The “concentration” of water is referred to as water potential. So osmosis is the movement of water from a higher water potential to a lower water potential across a membrane.
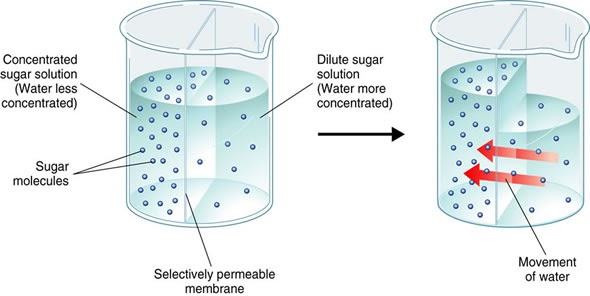
When does Osmosis occur?
For osmosis to occur it is essential that there is a semi-permeable membrane separating two environments with a different solute concentration. The solute must be unable to cross the membrane (molecules too big), but the water molecules are free to pass through and lead to an equilibrium. In the above image, the right side of the beaker has a higher water potential than the left side, so water moves in from right to left.
