Once the right environment is set up (often this means 37 deg. Celsius, shaking the flasks to introduce oxygen bubbles into the solution and optimise growth, leaving plates incubating overnight, etc.), growth can finally be monitored. There are many ways of doing this, such as cell counts, dilution plating, mass and optical methods that detect turbidity.
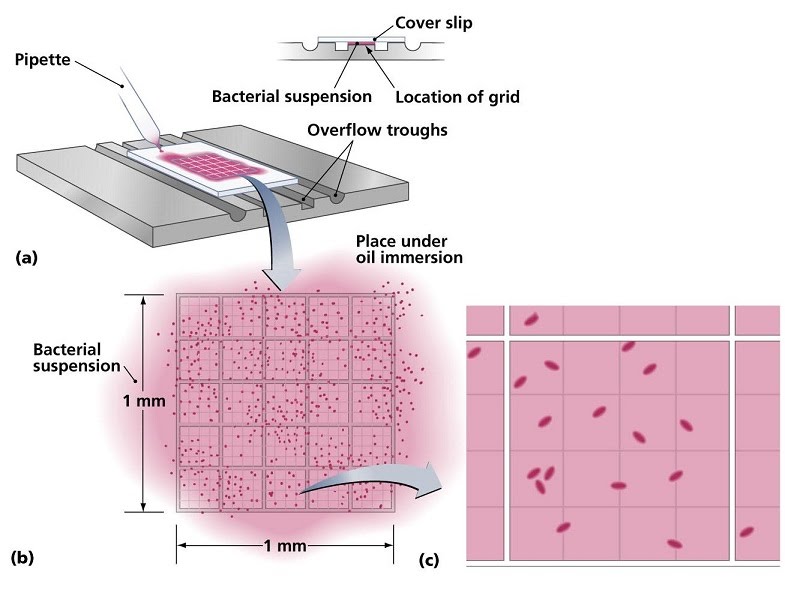
Cell counts involve pipetting a small volume from a liquid culture under a slide with a grid and looking at it under a microscope. The smaller sections of the grid can be used to count the number of cells, then multiply it by the number of sections and by the factor corresponding to the volume taken from the main solution, to obtain the total number of cells present.
For example, if we count 15 cells in one of 25 grid sections from a 10 microlitre sample of a total volume of 10 mililitres (there are 10,000 microlitres in 10 mililitres, so our sample is 10,000 / 10 = 1,000 times less than the total volume), we would compute:
15 cells x 25 sections x 1,000 = 375,000 cells in our total volume
Another method of measuring your cultured microorganism is dilution plating. This is done when the initial amount is unknown, as well as to find out various things about the organism. Sequential agar plates of increasingly diluted microorganism samples are set up, and the emerging colonies are counted (usually grown overnight). Colonies form from just a single or few cells, so are good for tracking growth and…
