Carbohydrate Digestion
Introduction
Glycosidic bonds
Reducing and non-reducing
Benedict’s reagent
Introduction
Carbohydrates as well as proteins are polymers and contain only a few different types of atom. In the case of carbohydrates, the basic molecular units are called monosaccharides – these are the monomers. (mono = single; poly = multiple; saccharide = sugar)
α (alpha) glucose is the most important monosaccharide to learn, as you need to be able to draw it:
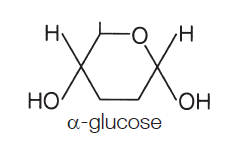
The points where the lines intersect each symbolise a carbon (C) atom. You need not show those. The figure above is taken from the specification itself, so take it as a good guide. So the monosaccharide alpha glucose (commonly, just glucose) somehow becomes a polysaccharide. This is achieved by condensation reactions, and the bonds formed are called glycosidic bonds.
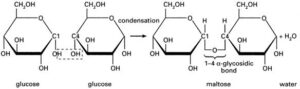
You should be able to draw this. The resulting molecule, maltose, is a disaccharide (two monomers). If you keep adding glucose molecules to the chain, you get… *drum roll please* …


