What are inorganic Ions?
Nitrate
Phosphate
Introduction
Inorganic ions like nitrate (NO3–), calcium (Ca2+), magnesium (Mg2+)and phosphate (PO43-) are key components of molecules in living things. Here are a few examples of where they can be found and what their role is within plants.
Nitrate ions are extracted by plants from the soil, and their nitrogen atoms used for other things. There are of course the nitrogenous bases in DNA (adenine, guanine, cytosine and thymine) as well as amino acids – hence amino acids. Get it get it.
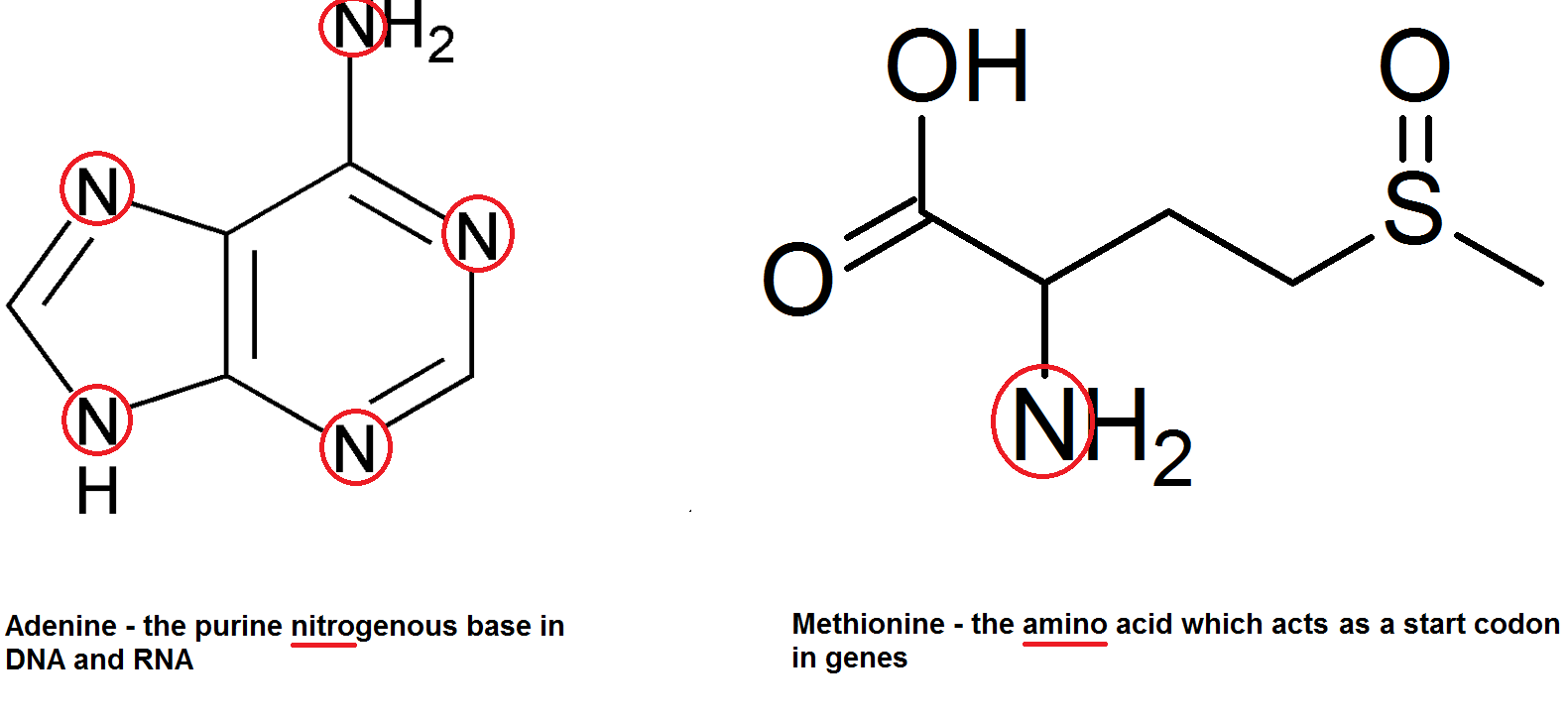
This of course applies to all life since all life does have DNA (or RNA) and amino acids.
Calcium ions have a key role in calcium pectate which I have just googled. I’m afraid what I found is far too hilarious to ignore:
“Calcium pectate, a pectin fiber that adds crispness to fruits and vegetables, also has potent cholesterol-lowering properties.”
Crispness. There you go. Crunchy kale. Crisp apples. Tooth-chipping swede. Crunch.
Anyway, back to the calcium pectate. The calcium ions bind to pectin (a carbohydrate) in plant cell walls creating a pectate salt, and contribute to its strength and stability. It’s present in all fruit and vegetables.
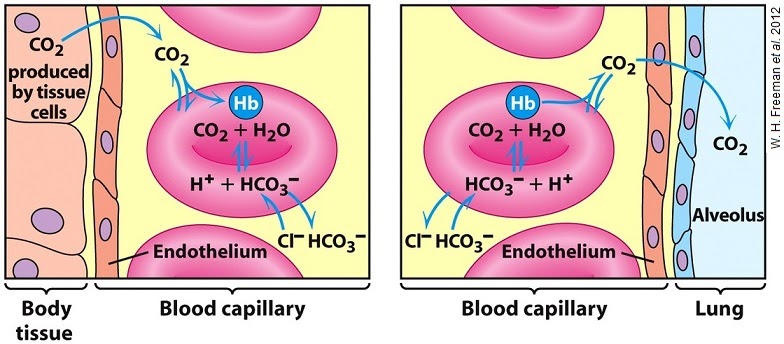
Hydrogen carbonate acts as an intermediary product between CO2 released from cellular respiration and water, and its reverse reaction releases CO2 back in the lungs for expiration.
Potassium, alongside sodium, are key ions in metabolism as they maintain concentration gradients in nerve signals, glucose….
